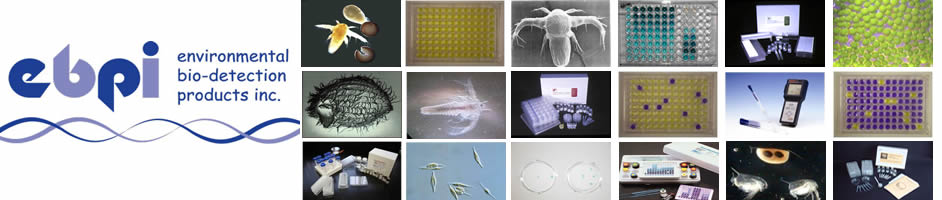
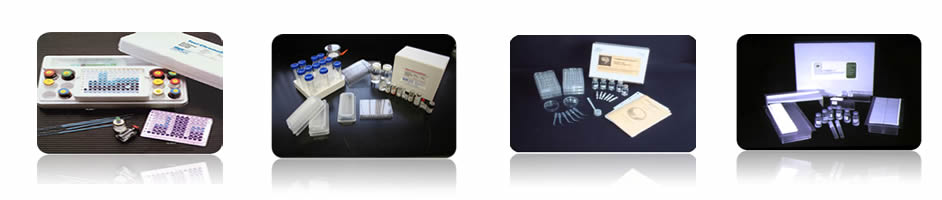
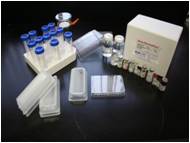
| Muta-ChromoPlate Kit |
 |
 |
 |
|
|
 |  |  |  |  |
Principle
The Muta-ChromoPlateTM kit is based on the most generally used and obacterial reverse-mutation test, known as the 'Ames Test' (Ames et al., 1975, Mutation Research 31:347).
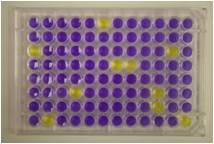
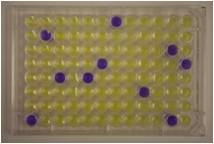
The test employs a mutant strain, or several strains, of Salmonella typhimurium, carrying mutation(s) in the operon coding for histidine biosynthesis. When these bacteria are exposed to mutagenic agents, under certain conditions, reverse mutation from amino acid (histidine) auxotrophy to prototrophy occurs.
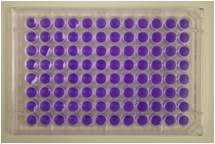
Traditionally, reverse-mutation assays have been performed using agar plates. An alternate assay performed entirely in liquid culture is the 'Fluctuation Test' based on multiple yes/no colour endpoints known as the Muta-ChromoPlate kit.
For more information please download EBPI's handout on the Ames Reverse Muta-ChromoPlate test kit.
Characteristics
The Muta-ChromoPlateTM kit is generally more sensitive than the pour-plate assay, because it allows testing of higher concentrations of sample (up to 75% v/v). The assay procedure is simple and requires minimal training. Consumable components are provided with ready-to-use and step-by-step instructions. "Instructions for Use" are provided with the basic kit. The only equipment required are a 37 degree Celsius incubator and a single and a multi-channel micropipettor.
S9 and Activation of Pro-Mutagens
S9 is a crude liver enzyme extract that can, under certain conditions, convert materials without any genotoxic activity to active genotoxic entities. The chemical process involved may be different for different materials. In addition, the lifetime of the activated moieties is extremely variable: some may be extremely short-lived. This is the reason for incubating the S-9 with the bacteria and the tested material at the same time during the assays.
Applications
Testing of pharmaceuticals for mutagenic activity
Testing of industrial effluents for presence of possible mutagenic compounds
Screening of municipal discharges for possible routine presence or spills of mutagenic compounds
Screening of surface and/or groundwater for mutagenic residues
Screening of potable water supplies for the presence of chemicals with mutagenic potential
Screening of water soluble air pollutants for mutagenic agents
Evaluation of pure or complexed raw mixtures for potential mutagenicity
A convenient and easy to use teaching tool for university and college laboratories
The Muta-ChromoPlateTM Ames Test kit is designed to be user friendly - removing potential contamination concerns.
Strains and controls as outlined in OECD Guideline for Testing of Chemicals 471
Strains of bacteria which can be included in the tests kits are TA97a TA98 TA100 TA1535 and WP2
Click here for a visual understanding on how the Reverse Mutation Assay is preformed